crunchbase news
The Food and Drug Administration just cleared the first surgical robot from a company in Taiwan.
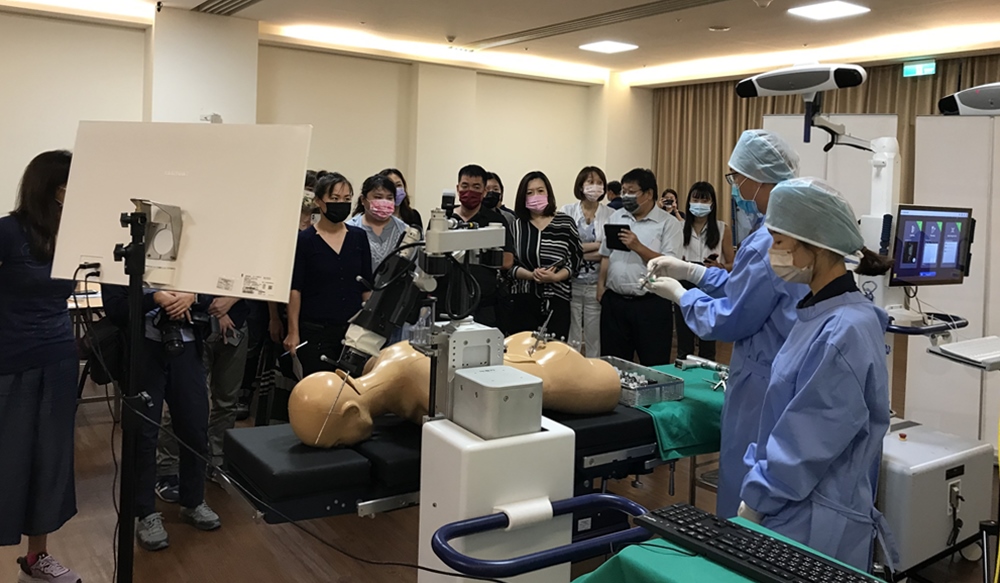
Taiwan-based Point Robotics announced on Tuesday it received FDA clearance for its Kinguide Robotic-Assisted Surgical System, a handheld robot that can be used in orthopedic surgeries.
The system creates 2D and 3D models of the anatomy and pinpoints where there needs to be a drill point. The robot acts like a “hand” for the surgeon to attach different surgical tools to and use for different procedures. It also balances shaky hands and guides the surgeon to make precise drills into the bone and add screws, making the process as minimally invasive as possible.
Point Robotics’ watershed FDA clearance is a large badge for Taiwan’s small health care and biotech industry. Funding in the space saw a 176.6% increase between 2019 and 2020, according to Crunchbase data, and went to only nine startups.
The next step for Point Robotics is to apply the surgical system to other types of spinal procedures like herniated disc decompression. It will also start pursuing approval in Europe and China.
The 6-year-old company raised $18 million in Series A funding in 2020 from Taiwania Capital, Translink Capital and through government-assisted funding. In January it raised an undisclosed sum of funding.
Read more details:
Point Robotics’ Surgical Robot Is Taiwan’s First FDA-Cleared

